When you hear the word generics, you probably think of cheap pills that work just like the brand-name version. But when it comes to biologics, that’s not the whole story. Biologics aren’t simple chemicals. They’re made from living cells-like proteins, antibodies, or vaccines-and that makes them incredibly complex. So when a cheaper version comes along, it’s not called a generic. It’s called a biosimilar. And the cost difference? It’s massive.
What’s the real price gap?
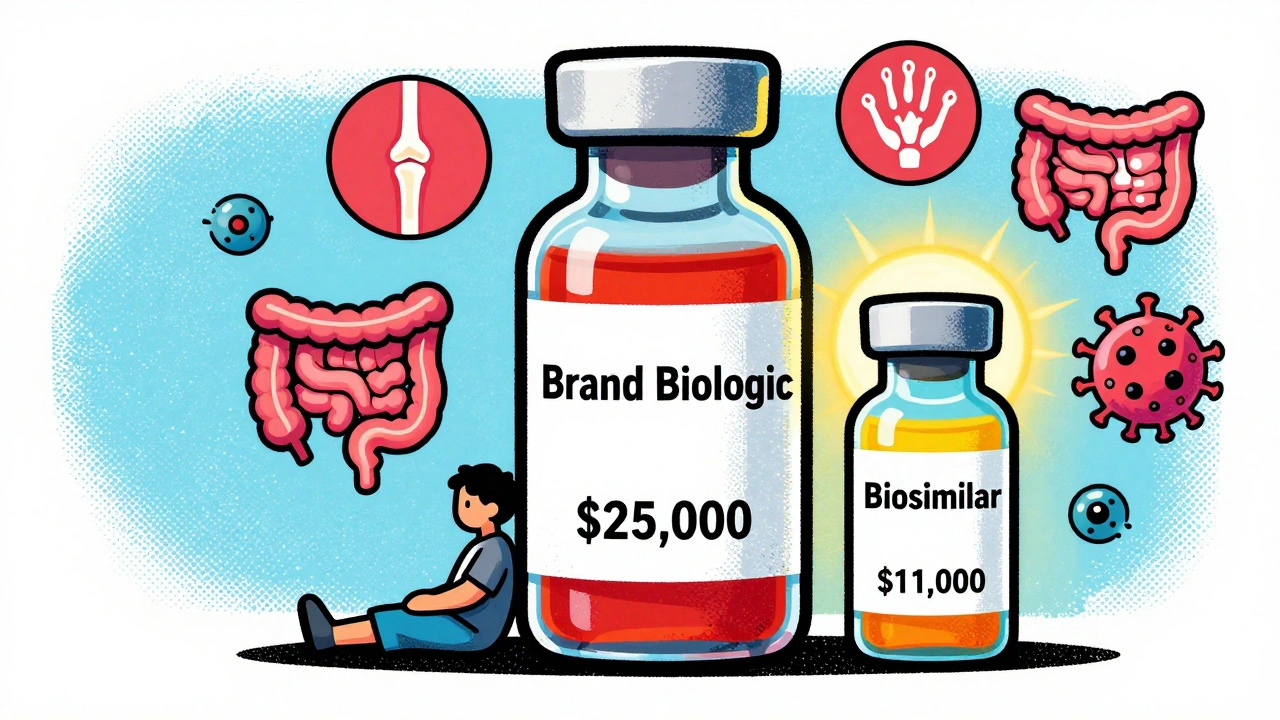
In 2025, the average 30-day prescription for a brand-name biologic cost $2,104. The biosimilar version? $919. That’s more than half off. For patients on long-term treatments-like for rheumatoid arthritis, Crohn’s disease, or cancer-those savings add up fast. A year’s supply of a brand biologic can hit $25,000. The biosimilar? Around $11,000. That’s $14,000 saved per person, every year.And it gets better. Once a biosimilar enters the market, the original brand often drops its price too. After biosimilars hit the market for Humira (adalimumab), the manufacturer cut its price by 33%. That’s not just a win for patients-it’s a win for insurers, hospitals, and taxpayers.
Take Humira. In 2022, it made $21.2 billion globally. Its U.S. list price? Around $80,000 per patient annually. By mid-2025, biosimilars captured 65% of the market. One of them, Hyrimoz by Sandoz, was already used by 14% of patients. And they’re all priced at least 80% lower than the original. That’s not a discount. That’s a revolution.
Why aren’t more people using biosimilars?
You’d think with savings this big, everyone would switch. But here’s the catch: the system isn’t built for it.Pharmacy Benefit Managers (PBMs)-the middlemen who negotiate drug prices for insurers-often lock in deals that favor the expensive brand. They get big rebates from the original manufacturers. That means even if a biosimilar costs half as much, the PBM might still push the pricier option because they make more money from it. Patients don’t see the rebate. They just see a higher copay.
Then there’s the patent game. Brand companies file dozens, sometimes hundreds, of patents on tiny variations of their drug-like changing the packaging, delivery method, or minor formulation tweaks. These aren’t real innovations. They’re legal shields. And they delay biosimilars for years. One analysis found that for every biologic that loses its main patent, it takes an average of 10 years before a biosimilar even gets approved.
Even when a biosimilar is approved, doctors and patients are hesitant. Many still believe biosimilars are “lesser.” But the FDA says they’re not. Every biosimilar must prove it works just as well and is just as safe as the original. No exceptions. No shortcuts. The FDA has approved 76 biosimilars as of October 2025. Not one has been pulled for safety issues.

Who’s saving money-and who’s not?
The savings aren’t just theoretical. Since 2015, biosimilars have saved the U.S. healthcare system at least $36 billion, according to DrugPatentWatch. Other estimates go as high as $56 billion. In 2024 alone, $12.4 billion to $20 billion was saved. That’s enough to cover free insulin for millions of people, or fund cancer screenings for hundreds of thousands.But here’s the irony: biosimilars make up less than 20% of the biologic market. Meanwhile, traditional generics-simple pills like metformin or lisinopril-make up 90% of all prescriptions but only 13% of total drug spending. Why? Because generics have multiple competitors. When five companies make the same pill, the price crashes. Biosimilars? Often just one or two enter the market at first. That’s not enough to force big price drops.
Patients with private insurance often pay 23% less out of pocket with biosimilars. But those on Medicare or Medicaid? Not always. Some plans still require prior authorization, step therapy, or don’t cover biosimilars at all. That’s not because they’re unsafe. It’s because the system hasn’t caught up.
What’s changing-and what’s next?
The FDA is finally stepping in. In September 2025, they released new draft guidance to cut the time and cost of developing biosimilars. No more redundant clinical trials. No more unnecessary testing. That could slash development costs from $100-250 million down to $50 million. More companies will enter the market. More competition. Lower prices.The Biden administration’s Biosimilars Action Plan aims to fix reimbursement rules so insurers aren’t punished for choosing cheaper options. And lawmakers are pushing bills to ban “patent thickets” that block competition.
By 2030, analysts predict biosimilar use will jump from 15-20% to 35-40%. That could mean $125 billion in annual savings. Imagine what that could do for diabetes care, autoimmune diseases, or cancer treatment. More people could get the drugs they need. Fewer would skip doses because of cost. Fewer would go bankrupt.
What you can do right now
If you’re on a biologic, ask your doctor: “Is there a biosimilar available for my drug?” Don’t assume your prescription is set in stone. Your pharmacist can also check. Sometimes, switching is as simple as a new prescription.If you’re on Medicare, check your plan’s formulary. Some plans now cover biosimilars with lower copays. Call them. Ask for a list of covered biologics and their biosimilar alternatives.
And if you’re paying out of pocket? Compare prices. A biosimilar might cost $100 a month instead of $700. That’s not a small difference. It’s life-changing.


Biosimilars are the silent revolution nobody’s talking about but everyone’s benefiting from. I work in pharmacy and I’ve seen patients cry when they switch from Humira to Hyrimoz and their copay drops from $800 to $80. It’s not magic-it’s science, and the FDA didn’t cut corners. These aren’t knockoffs. They’re life-savers. 🙌
Patent thickets are legal fraud. Pharma companies file 200 patents on a drug just to delay biosimilars. It’s not innovation. It’s rent-seeking. The system is broken. Fix it or shut up.
It’s fascinating how people act like biosimilars are some radical new idea, when in reality, the entire pharmaceutical industry is built on exploiting regulatory loopholes, patent games, and patient desperation. The fact that we’re even having this conversation reveals how deeply broken our healthcare system is. People are dying because a corporation can’t be bothered to let competition exist. And yet, we’re told to be grateful for a 20% discount.
bro i just switched to a biosimilar for my crohn’s med and my wallet is crying tears of joy 😭 like i was paying $700/mo now it’s $90. my dr was like ‘oh yeah there’s one’ like it was no big deal. why is this not common knowledge???
This is the most important healthcare story nobody’s talking about. The fact that we’re saving $12B+ a year with biosimilars and still only using them for 20% of biologics? That’s not a market failure-it’s a policy failure. We need to incentivize pharmacies to push these, not punish them. And we need to ban those sneaky patent extensions. 🙏
Of course the PBMs are the real villains here. They’re the reason you pay more even when the drug is cheaper. They’re not intermediaries-they’re parasites. And doctors? They don’t even know the difference between a biosimilar and a generic. They just prescribe what’s in the formulary. Blame the middlemen, not the patients.
Let me guess-the ‘revolution’ is just another corporate PR stunt. Biosimilars are just rebranded generics with a fancy name so Big Pharma can pretend they’re not ripping us off. And don’t tell me about FDA approval-I’ve seen how they greenlight things with 300-patient trials. Real medicine doesn’t work like this.
Oh wow, so now we’re supposed to be impressed that a drug costs $11k instead of $25k? That’s like being thrilled you got a $1000 discount on a $5000 yacht. Meanwhile, my insulin still costs $300. Biosimilars are a Band-Aid on a hemorrhage.
I’ve spent years watching patients choose between food and their biologics. The moment a biosimilar became available for my sister’s rheumatoid arthritis, she stopped skipping doses. She started walking again. That’s not a cost saving-it’s a restoration of dignity. The real tragedy isn’t the price tag. It’s that it took this long for anyone to care.
India makes biosimilars cheap. US pays more. Why? Because system rigged. Simple.
in india we get biosimilars for 1/10th the price and they work fine. i wish more americans knew this. its not about science its about profit. sad.
Ohhh so now we’re supposed to be ‘grateful’ for a drug that’s still 10x more than a regular generic? Cute. Next you’ll tell me that paying $900/month for a biologic is ‘affordable’ if you’re rich. 🙄
Biologics = billion-dollar industry. Biosimilars = threat. PBMs = enablers. Patients = collateral. The math is clear. It’s not about health. It’s about control.
While the aggregate savings figures are indeed statistically significant, one must interrogate the structural incentives embedded within the value chain. The current reimbursement architecture, particularly as mediated by Pharmacy Benefit Managers, creates perverse incentives that actively disincentivize biosimilar adoption at the point of care. Moreover, the regulatory pathway, though improved, still imposes disproportionate burdens on manufacturers relative to the marginal clinical benefit conferred. The FDA’s recent guidance, while commendable, remains insufficiently calibrated to the scale of market distortion. A true paradigm shift would require dismantling the rebate system entirely and implementing reference pricing akin to European models. Until then, we are merely rearranging deck chairs on the Titanic.