Every year, hundreds of thousands of people end up in hospitals not because their illness got worse, but because the medicine meant to help them made things worse. These are called adverse drug reactions-unexpected, harmful side effects that can range from a nasty rash to life-threatening organ damage. For many, it’s not bad luck. It’s genetics.
Why Your Genes Matter More Than Your Prescription
You might take the same dose of a drug as your neighbor, but your body handles it completely differently. That’s because of small differences in your DNA. These variations affect how your liver breaks down medications, how your kidneys clear them, and even how your cells respond to them. One person might metabolize a drug too fast, making it useless. Another might process it too slow, causing toxic buildup. This isn’t rare. In fact, the PREPARE study found that 93.5% of people have at least one gene variant that changes how they respond to common medications.How Pharmacogenetic Testing Works
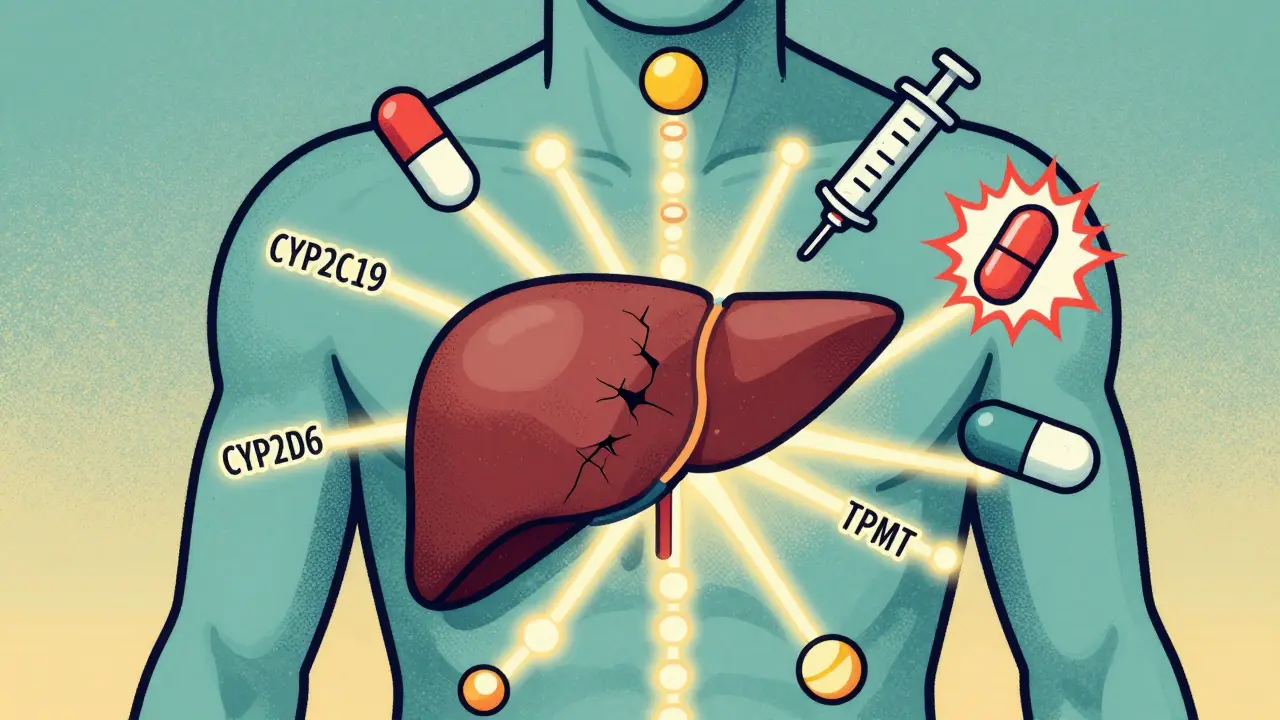
Pharmacogenetic testing looks at your DNA to predict how you’ll react to certain drugs. It’s not about predicting disease-it’s about predicting drug response. A simple cheek swab or blood sample is sent to a lab that checks for specific gene variants linked to known drug reactions. The most common genes tested include:- CYP2C19: Affects clopidogrel (Plavix), antidepressants, and proton pump inhibitors
- CYP2D6: Influences codeine, tamoxifen, and many antidepressants
- TPMT: Critical for azathioprine and mercaptopurine, used in autoimmune and cancer treatments
- HLA-B*1502: Flags high risk of severe skin reactions to carbamazepine, especially in people of Asian descent
- SLCO1B1: Predicts muscle damage from statins like simvastatin
The test doesn’t just say “yes” or “no.” It tells your doctor if you’re a poor, intermediate, normal, rapid, or ultra-rapid metabolizer. That changes everything. For example, if you’re a poor CYP2C19 metabolizer, clopidogrel won’t work for you. Your doctor can switch you to prasugrel or ticagrelor right away-before you have a heart attack.
The Proof: What the Science Shows
The biggest evidence comes from the PREPARE study, published in The Lancet in 2023. Researchers tested nearly 7,000 patients across seven European countries before giving them common prescriptions. They used a 12-gene panel that covered over 100 medications. The result? A 30% drop in serious adverse drug reactions compared to patients who got standard care. That’s not a small win. It’s the difference between 1 in 10 people getting hurt and 1 in 14.Some reactions are almost entirely preventable. HLA-B*1502 testing before giving carbamazepine cuts the risk of Stevens-Johnson syndrome by 95% in Asian populations. TPMT testing before azathioprine reduces life-threatening bone marrow suppression by 78%. These aren’t theoretical numbers. They’re real outcomes from real patients.
How It Compares to Other Methods
Doctors have used other tools to avoid bad reactions. Therapeutic drug monitoring checks drug levels in your blood after you’ve already taken it. But that’s like checking your car’s oil after the engine seized. Pharmacogenetic testing is like reading the manual before you start the engine.Clinical risk scores-based on age, weight, kidney function-help, but they miss the genetic piece. Two people with identical risk scores can have completely different reactions because of their DNA. Pharmacogenetic testing adds precision where guesswork used to rule.
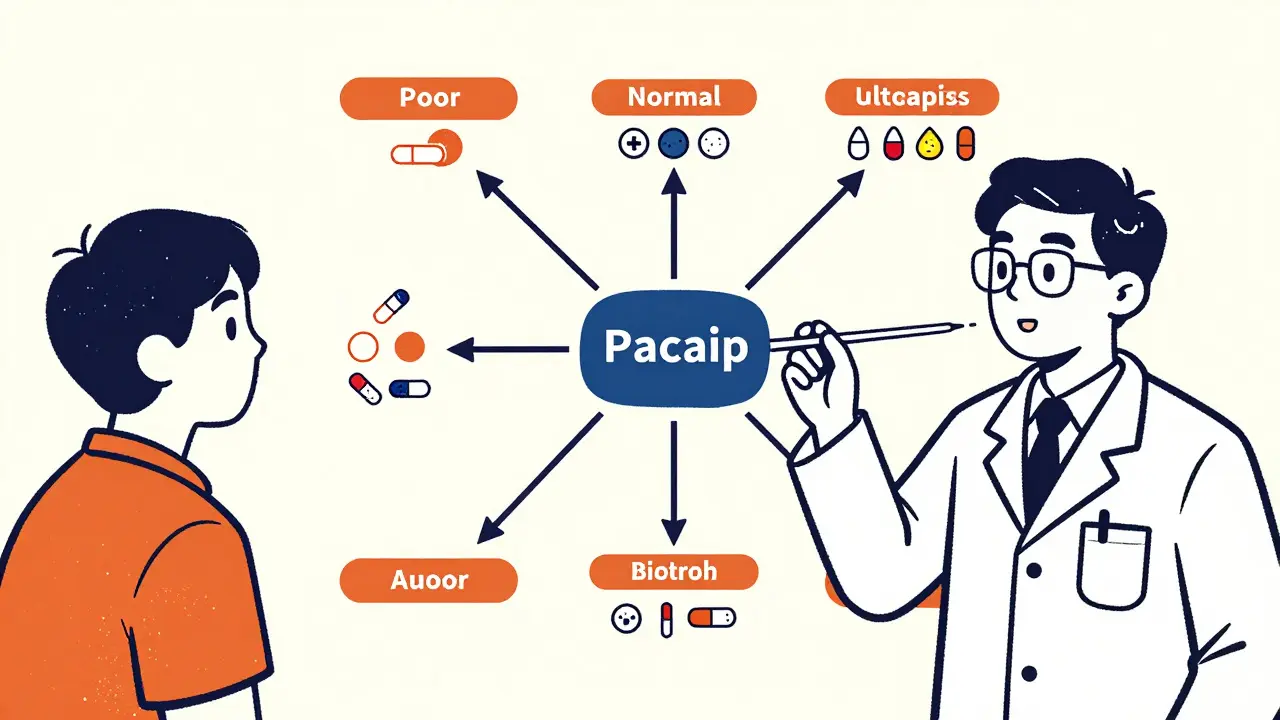
Who Benefits the Most?
It’s not just for people with complex conditions. While oncology and psychiatry lead in adoption, the real opportunity is in primary care. Think about it: 1 in 5 adults takes five or more medications. The more drugs you take, the higher your risk of a bad interaction. A patient on statins, an antidepressant, and a blood thinner? Their chances of a hidden gene-drug conflict are high. Testing before prescribing could prevent hospital visits, emergency care, and even death.Psychiatric patients see especially strong results. One trial with 685 people showed a sharp drop in side effects like dizziness, nausea, and fatigue after switching to genotype-guided treatment. Patients felt better faster-and stayed on their meds longer because they weren’t being poisoned by the treatment.
Cost and Accessibility
The test costs between $200 and $500 in the U.S., depending on the panel. That sounds expensive until you compare it to the cost of an ADR. The NHS estimates adverse drug reactions cause 7% of all hospital admissions-roughly £500 million a year in avoidable spending. One study found pharmacogenetic testing pays for itself in under 18 months by cutting ER visits and hospital stays.In the U.S., Medicare covers testing for certain high-risk pairs like CYP2C19 before clopidogrel and TPMT before thiopurines. In Europe, countries are rolling out nationwide programs based on the PREPARE study results. The European Commission just committed €150 million to scale this up by 2027.
Barriers to Adoption
Despite the evidence, adoption is slow. Only 18% of primary care practices in the U.S. use pharmacogenetic testing. Why? Three big reasons:- Clinicians don’t know how to use the results. A survey found only 37% of doctors feel confident interpreting pharmacogenetic reports.
- Electronic health records don’t alert them. If the test result doesn’t pop up in the prescription system, it’s useless.
- There’s no clear pathway. What do you do if someone is an intermediate metabolizer for three different drugs? Guidelines exist (like CPIC and DPWG), but they’re not always integrated into daily practice.
Implementation takes time. The University of Florida spent $1.2 million to build their system but cut ADR-related ER visits by 75% within two years. Success requires training, tech integration, and clear protocols-not just a test kit.

The Future: Faster, Cheaper, Broader
Right now, results take 24 to 72 hours. But new point-of-care PCR tests are in development that could give results in under an hour-and cost as little as $50 by 2026. That means testing could happen during a clinic visit, not weeks before.Researchers are also moving beyond single genes. Polygenic risk scores, which combine dozens of small genetic signals, are showing 40-60% better accuracy in predicting drug response than single-gene tests. This is especially important for complex drugs like antidepressants, where multiple genes play a role.
And the data is getting more inclusive. Until recently, most pharmacogenetic studies were done in European and East Asian populations. New research from the NIH is adding 126 new gene-drug links from African and Indigenous groups, making testing more accurate for everyone.
What Patients Need to Know
If you’re on multiple medications, have had bad side effects before, or are about to start a new drug-ask your doctor about pharmacogenetic testing. Most patients (85%) say they’d be willing to take the test if their doctor recommends it. But only if they understand it’s not about your future health risks-it’s about making your current treatment safer.Privacy concerns are real. About one-third of people worry about how their genetic data will be used. Reputable labs store results securely and only share them with your care team. Your insurance can’t deny coverage based on these results-thanks to GINA, the federal law that protects against genetic discrimination in health insurance and employment.
What Comes Next
By 2026, 87% of major U.S. academic hospitals plan to offer preemptive pharmacogenetic testing. Europe is following close behind. This isn’t science fiction. It’s the next step in safe, personalized care.Medicine has spent decades trying to guess the right dose. Now, we can know. And that’s not just better-it’s life-saving.
Is pharmacogenetic testing covered by insurance?
In the U.S., Medicare and some private insurers cover testing for specific high-risk drug-gene pairs, like CYP2C19 before clopidogrel or TPMT before azathioprine. Coverage for broader panels varies. In Europe, national health systems are increasingly funding preemptive testing based on the PREPARE study results. Always check with your provider or insurer before testing.
How long does it take to get results?
Most clinical labs deliver results in 24 to 72 hours. New point-of-care tests are being developed that could give results in under an hour, making it possible to use the data during a doctor’s visit. Turnaround time depends on the lab, the test panel, and how the sample is processed.
Can pharmacogenetic testing predict all drug side effects?
No. It only predicts reactions linked to known gene-drug interactions-currently around 329 pairs recognized by the FDA. Many side effects are caused by non-genetic factors like age, liver disease, or drug interactions. Pharmacogenetic testing reduces risk, but it doesn’t eliminate all possibilities. It’s one tool in a larger safety strategy.
Do I need to get tested more than once?
No. Your genes don’t change. A single test gives you lifelong information about how your body processes medications. Once you’ve been tested, the results can be used every time you’re prescribed a new drug. That’s why preemptive testing-done before any medication is started-is so powerful.
What if my doctor doesn’t know about pharmacogenetic testing?
It’s not uncommon. Only 37% of physicians feel confident interpreting results. You can ask for a referral to a pharmacogenetics specialist, a clinical pharmacist, or a personalized medicine program at a major hospital. Many labs also provide free educational resources for providers. Bring printed guidelines from CPIC or DPWG to your appointment to help start the conversation.

Been using this in my clinic for a year now. We tested patients on statins and SSRIs first. The drop in side effect complaints was wild. One guy stopped his antidepressant because he felt like a zombie-turned out he was an ultra-rapid CYP2D6 metabolizer. Switched him to a different class, and now he’s hiking weekends. Simple test, huge payoff.
bro i got tested last year after my cousin had a bad reaction to plavix and holy crap it changed everything. my doc just looked at the report like it was alien tech but i printed out the cpic guidelines and he’s now my favorite doctor. genetics ain’t magic but it’s way better than guessing
This is just another way for big pharma and labs to profit. We’ve been prescribing drugs for 100 years without this. If your body reacts badly, stop taking it. Simple. Why do we need a $500 DNA test to tell us what side effects are? We already have warning labels.
While the science is compelling, one must consider the cultural and systemic barriers in developing nations. In India, where access to basic healthcare remains inconsistent, investing in pharmacogenomics is a luxury few can afford. The infrastructure required-labs, trained personnel, EHR integration-is absent in most rural clinics. Until equity in healthcare is addressed, such innovations remain theoretical for the majority.
93.5% have a variant? That’s not precision medicine. That’s a universal red flag. If nearly everyone has a dangerous gene, then the drugs themselves are the problem-not the genes. This is a cover-up. The pharmaceutical industry profits from treating side effects, not preventing them. They don’t want you to know this.
so you’re telling me we’re all just broken machines and science is here to fix us with more tech? what’s next, gene therapy for being bad at math? this is just another way to make people feel like their bodies are defective. maybe the real issue is we’re overmedicating everything.
Imagine if we tested people for how they handle caffeine before giving them coffee. ‘Oh, you’re a slow metabolizer? Here’s a decaf.’ We’ve been doing this with drugs for decades like we’re playing Russian roulette with prescriptions. Time to stop being lazy and start being smart. This isn’t sci-fi-it’s basic responsibility.
my mom got tested after she almost died from a statin. they said it was ‘genetic’ and i cried. but then i realized-why didn’t anyone tell us this before? why is this still optional? if my grandma had known, she might still be here. we’re not just talking science-we’re talking lives. and we’re failing.
I’ve been reading up on this for months. The real breakthrough isn’t just the genes-it’s the integration. Having the result pop up in the EHR when the doctor tries to prescribe simvastatin? That’s the game-changer. Right now, most docs get a 10-page PDF and just ignore it. We need alerts, decision trees, and mandatory training. It’s not about the test-it’s about the workflow. And yeah, the data inclusion from African and Indigenous populations? Long overdue. We’ve been using Euro-centric data for too long. It’s not just ethical-it’s scientifically inaccurate.